Returns Reconciliation and Destruction
Returns Reconciliation and Destruction services tailored to the accountability needs of your clinical trial.
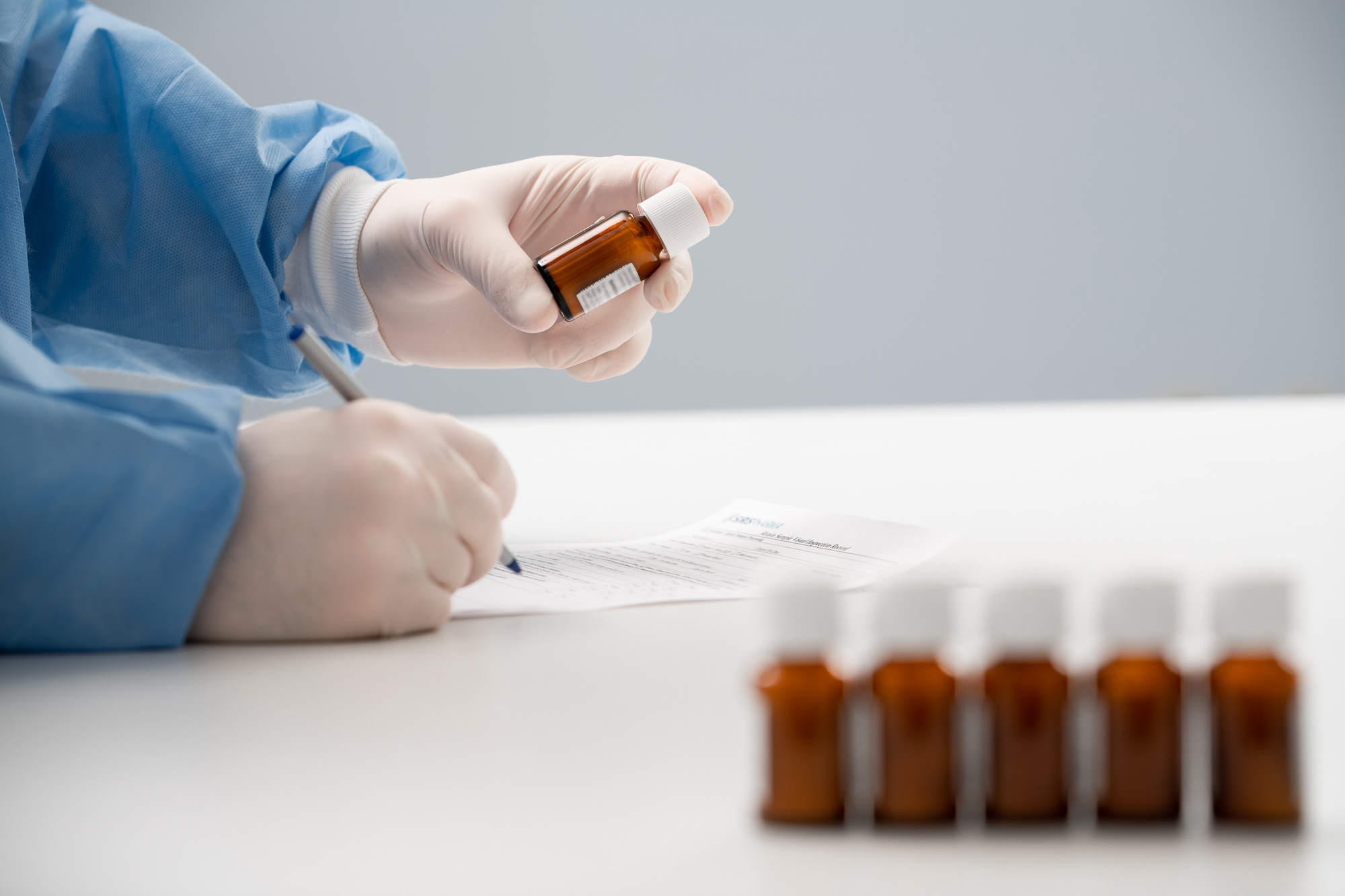
Drug Accountability
Federal guidelines require sponsors and investigators to maintain 100% accountability of all investigational drug products used in a clinical study. This means actively managing finished and/or discontinued clinical sites and maintaining records for the disposition of the drug, quantities, whether the drug was used or unused, etc.
The start of a clinical trial is often focused on getting study drugs packaged, distributed and administered to your first patient. While this focus is understandable, the end of your clinical trial and the accountability, returns reconciliation and destruction of used and un-used drug products can be an overlooked and challenging aspect of bringing closure to your clinical study.

Returns Reconciliation and Destruction Services
Siris offers comprehensive returns reconciliation and destruction services to help bring closure to your clinical trial. Our team will work closely with you to develop an appropriate reconciliation strategy that aligns with your clinical protocol and maintains compliance and safety measures. Whether you need a simple receipt and hold until destruction, or breaking down every kit and counting each individual tablet, Siris’ trained technicians will execute your reconciliation in compliance with cGMPs.
As your returned clinical supplies are received, Siris can store your returns at their appropriate temperature conditions if there is potential to re-use supplies, or at ambient temperatures to keep costs down. When your study is completed, Siris can also help you manage proper destruction of your drug products. Working with licensed waste facilities, Siris will profile drug products to ensure proper disposal and provide all documentation to show traceability.
Take the First Step With Siris
Schedule a 15-minute consultation